https://www.bayoocare.com/wp-content/uploads/sites/10/2023/05/MTL-23_Logo_coloured_RGB-300-dpi.png
1181
1831
Alexander Manger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Alexander Manger2023-05-09 13:10:532023-05-15 12:10:50BAYOOCARE @MedTecLive 2023
https://www.bayoocare.com/wp-content/uploads/sites/10/2023/05/MTL-23_Logo_coloured_RGB-300-dpi.png
1181
1831
Alexander Manger
https://www.bayoocare.com/wp-content/uploads/sites/10/2020/05/Bayoocare-300x95.png
Alexander Manger2023-05-09 13:10:532023-05-15 12:10:50BAYOOCARE @MedTecLive 2023News & Insights:
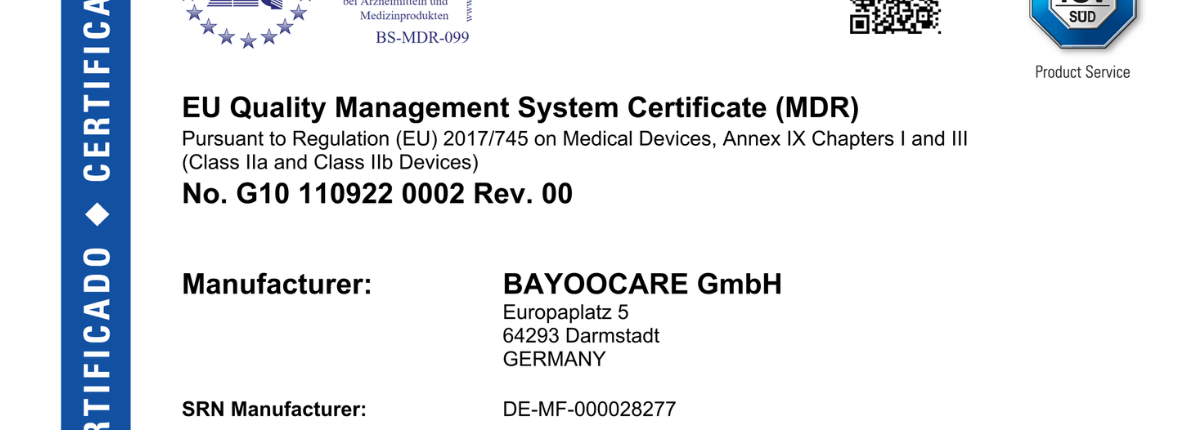
Here you’ll find in-depth articles on Regulatory Affairs – clearly structured, up to date, and practice-oriented. In our blog, we cover regulatory developments, guidance documents, and their concrete implications for manufacturers and distributors.
Stay informed: Sign up for our Regulatory Affairs newsletter and don’t miss important updates, deadlines, or new regulatory requirements.